Lipid Based Formulation
Softgels: Gelatin softgels, Seamless softgels and Vegicaps
For faster action and /or increased bioavailability, any drug delivery system that provides drug in solution, such as softgel capsules and seamless softgel capsules, has a head start on any technology based around particulate or amorphous drug powder. Vegicaps capsules allow higher temperatures to be used during filling that can increase the amount of API that can be used to be encapsulated, that would normally not be possible due to viscosity constraints.
Formulation Rationale:
a. Drug delivery systems: Liquid and semi-solid formulations of the compound in solution or suspension are often employed to improve bioavailability utilizing one of the systems listed below:
- • Lipid-based systems
- • Hydrophilic systems
- • Amphiphilic systems
b. Excipients: Listed below are the excipients frequently used in lipid-based softgel formulations:
1. Excipients in Liquid form include:
- 1. Oils: Corn Oil, Soybean Oil,Peanut Oil, Oleic Acid, Neobee M-5, Miglyol 812, Captex 355, Akomed-R and Labrafac CC
- 2. Surfactants (HLB<10): Miglyol 840, Captex 200, Capmul MCM, Maisine 35-1, Labrafil M 1944 CS, Labrafil M 2125 CS, SPAN 80, SPAN 20, Plurol Oleique, Crill 4, Neobee M-20 and Marlowet 4750
- 3. Surfactants (HLB>10): Polysorbate 80, Polysorbate 20, Labrasol, SPAN 20, Crillet 4, Softigen 767
2. Excipients in the Semi-Solid form include:
- 1. Waxes: Yellow Wax, Paraffin Wax, Microcrystalline Wax and Carnauba Wax
- 2. Fatty Alcohols: Cetyl Alcohol, Steryl Alcohol and Cetosteryl Alcohol
- 3. Hydrogenated Oils: Sterotex and Softisan 154
- 4. Surfactants (HLB<10): Imwitor 988, Imwitor 742, and Gelucire 43/01
- 5. Surfactants (HLB>10): Gelucire 44/14, Gelucire 50/13, Vitamin E TPGS, Cremophor RH 40, Cremophor EL, Labrafil M 2130 CS, Brij 96 and Myrj 45
c. Lipid Formulation Classification System: Based on the excipients in the formulation, the systems are classified as shown below
Table IV: Lipid Formulation Classification System
| Excipients in Formulation | Content of formulation (% w/w) | ||||
| Type I | Type II | Type IIIA | Type IIIB | Type IV | |
| Oils (Triglycerides or mixed mono and diglycerides) | 100% | 40-80% | 40-8 % | <20% | |
| Water Insoluble Surfactants (HLB < 12) | 20-60% | 0-20% | |||
| Water Soluble Surfactants (HLB > 12) | 20-40% | 20-50% | 30-80% | ||
| Hydrophilic cosolvents e.g. PEG, PyG…) | 0-40% | 20-50% | 0-50 % | ||
d. Increased solubilization of poorly water soluble (PWS) drugs in the G.I.T. is achieved and maintained by:
- • Dispersion (self-emulsifying systems)
- • Digestion (lipolysis)
Factors affecting membrane permeation are:
- • Passive transport through enterocytes
- • Passive transport around enterocytes (tight junctions)
- • Enterocyte-based active transport and metabolic processes (P-gp, cytochrome P 450, lipoproteins)
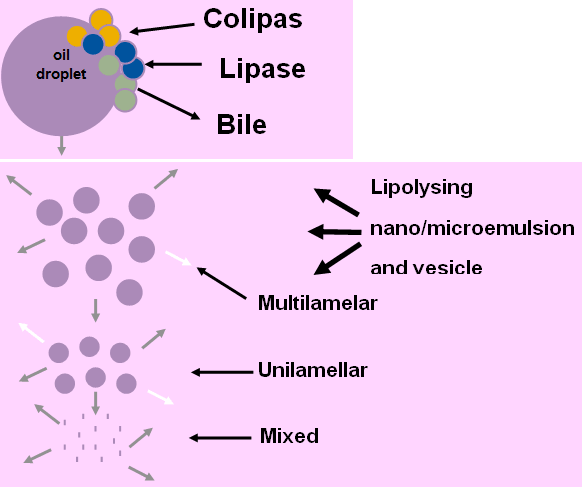
Digestion plays a significant role in the development of Lipid-Based Formulations: Fat Digestion or Lipolysis occurs primarily in the small intestine.
- • Pancreas Pancreatic lipase-colipase complex
- • 1 mole of triglyceride metabolised 1 mole of monoglyceride + 2 moles of free fatty acids
- • Liquid crystalline phase vesicles + mixed intestinal micelles
e. The Mechanism of Bioavailability Enhancement:
Fig. 2: Digestion or Lipolysis.
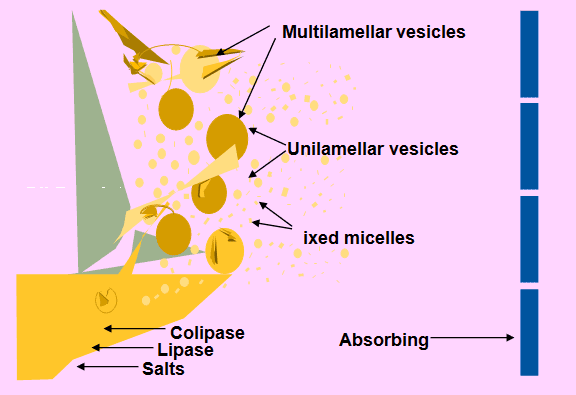
Fig 3: Mixed Micelles: The End Product of Lipolysis.
Unilamellar vesicles
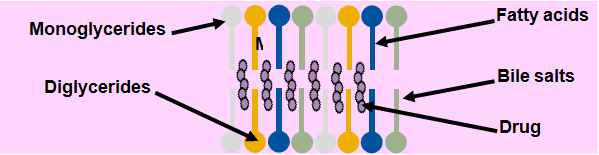
Disc shaped micelles
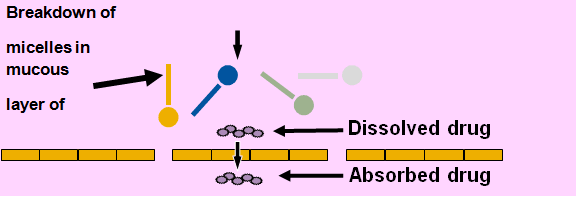
f Softgels for Self-Emulsifying Lipid-Based Formulations Undergoing Lipolysis:
Fig 4:
Formulation approaches:
• Formulation Strategies
- 1. Lipolyzing microemulsion preconcentrate
- 2. Lipid-based drug delivery systems
- 3. Nanocrystals
- 4. Solid solutions/dispersions
- 5. Solid-lipid nanoparticles
- 6. Inclusion complexes (cyclodextrins), etc.
• Dosage Form Options
- 1. Softgel capsules (gelatin or plant-based)
- 2. Hardshell capsules (gelatin, HPMC, plant-based; liquid or powder filled)
- 3. Oral solutions or suspensions
- 4. Tablets
1. A Lipolyzing microemulsion preconcentrate into a softgel results in:
- • Rapid gastric dispersion due to self-emulsifying properties
- • Maintain drug in solution using a solvent system which prevents precipitation
- • Digestible lipid composition to facilitate transfer of solubilized drug via mixed micelles
- • High drug concentration at the site of absorption
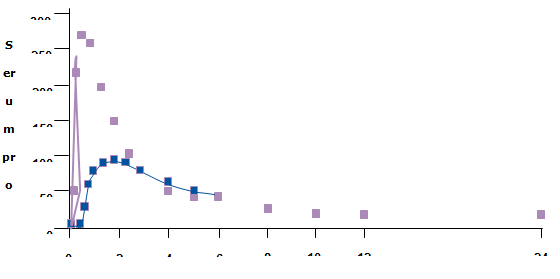
Case Study 2: Progesterone
Progesterone
Physiochemical Properties
- • High molecular weight (314.5)
- • Hydrophobic drug
- • Poor solubility in aqueous GI fluids (< 0.1 mg/ml in water)
Pharmacokinetic Properties
- • Poorly absorbed
- • Rapidly metabolized by the liver
- • Slow dissolution and absorption from oral suspension provides steady flow of drug to liver where it is extensively metabolized
Fig 5: Solubility of Progesterone in Pre-Digested Triglycerides and Lipolyzed Formulation.
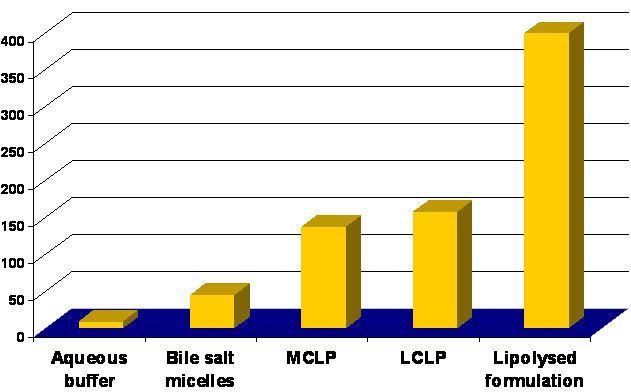
Formulation composed of 1g triglyceride / lipophilic surfactant / hydrophilic surfactant / co-solvent
Progesterone Relative Bioavailability from a Lipolyzing Solution and a Suspension Formulation
Fig 6: Serum Concentration versus Time Curves following Single Dose Administration of 200mg Progesterone in 12 Healthy Post-Menopausal Volunteers.